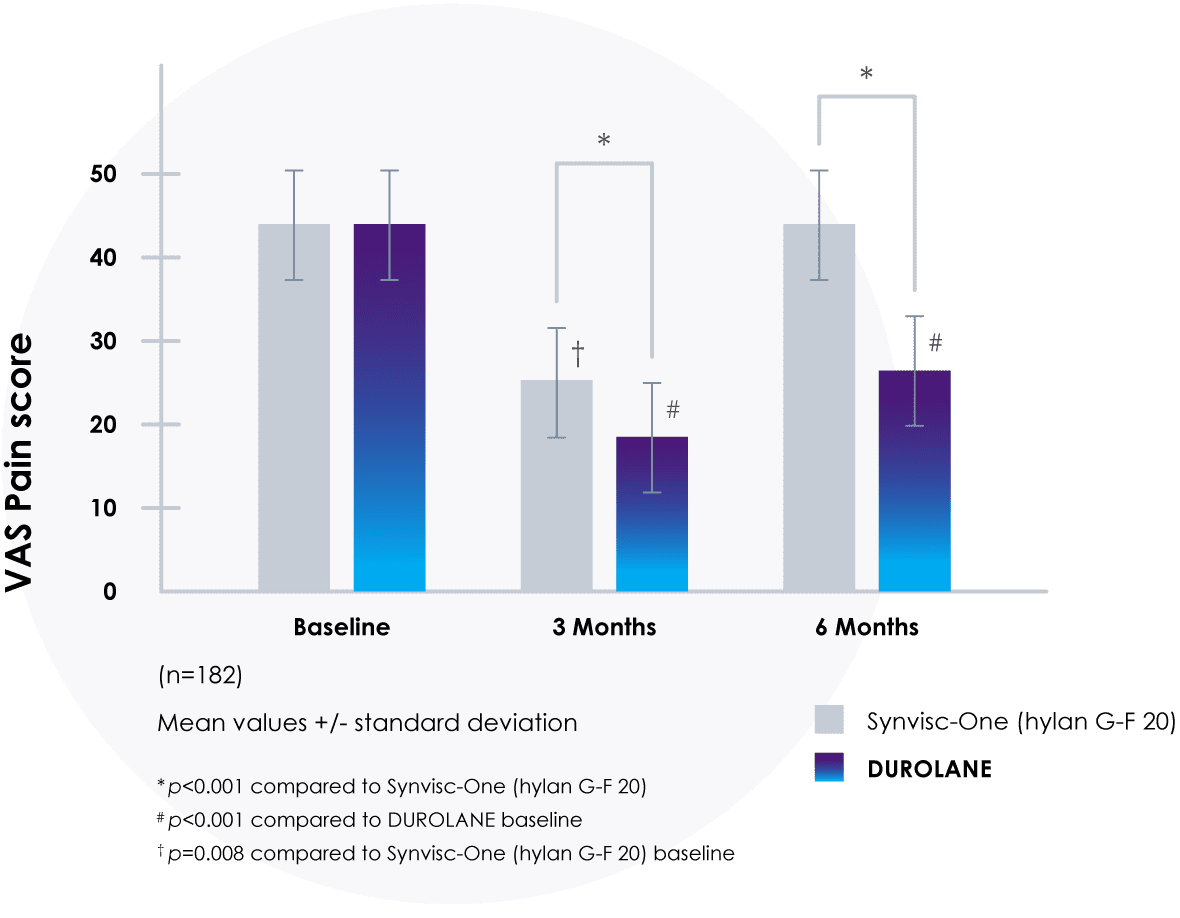
Compared directly to Synvisc-One®,‡25 DUROLANE reduced pain more effectively.
In a Level 1 study, there was a significantly greater reduction in visual analog scale (VAS) pain scores at 3 and 6 months with DUROLANE compared to Synvisc-One (hylan G-F 20) (p<0.001).‡25
DUROLANE showed:
- 41% lower VAS pain score at 6 months vs. Synvisc-One (hylan G-F 20)
- Only DUROLANE maintained a significant reduction vs. baseline
‡Some patients were treated with a three-injection Synvisc® regimen. A three-injection Synvisc regimen is equivalent to one injection of Synvisc-One.
Synvisc and Synvisc-One are registered trademarks of Genzyme Biosurgery.
Clinically Equivalent to Five-Injection Hyaluronic Acid Therapy.13,24
In a Level 1 study, one injection of DUROLANE:
- Produced pain reduction at 6 months that was noninferior to pain relief following five injections of the comparator hyaluronic acid
- 79% of DUROLANE-treated patients experienced improved pain control for up to 26 weeks§11
- Choose convenience without compromise and get powerful results of a single-injection
§Outcomes measured by WOMAC subscores. The primary outcome was the WOMAC pain responder rate, defined as at least 40% relative improvement and 5-point absolute improvement from baseline values at 12 weeks.
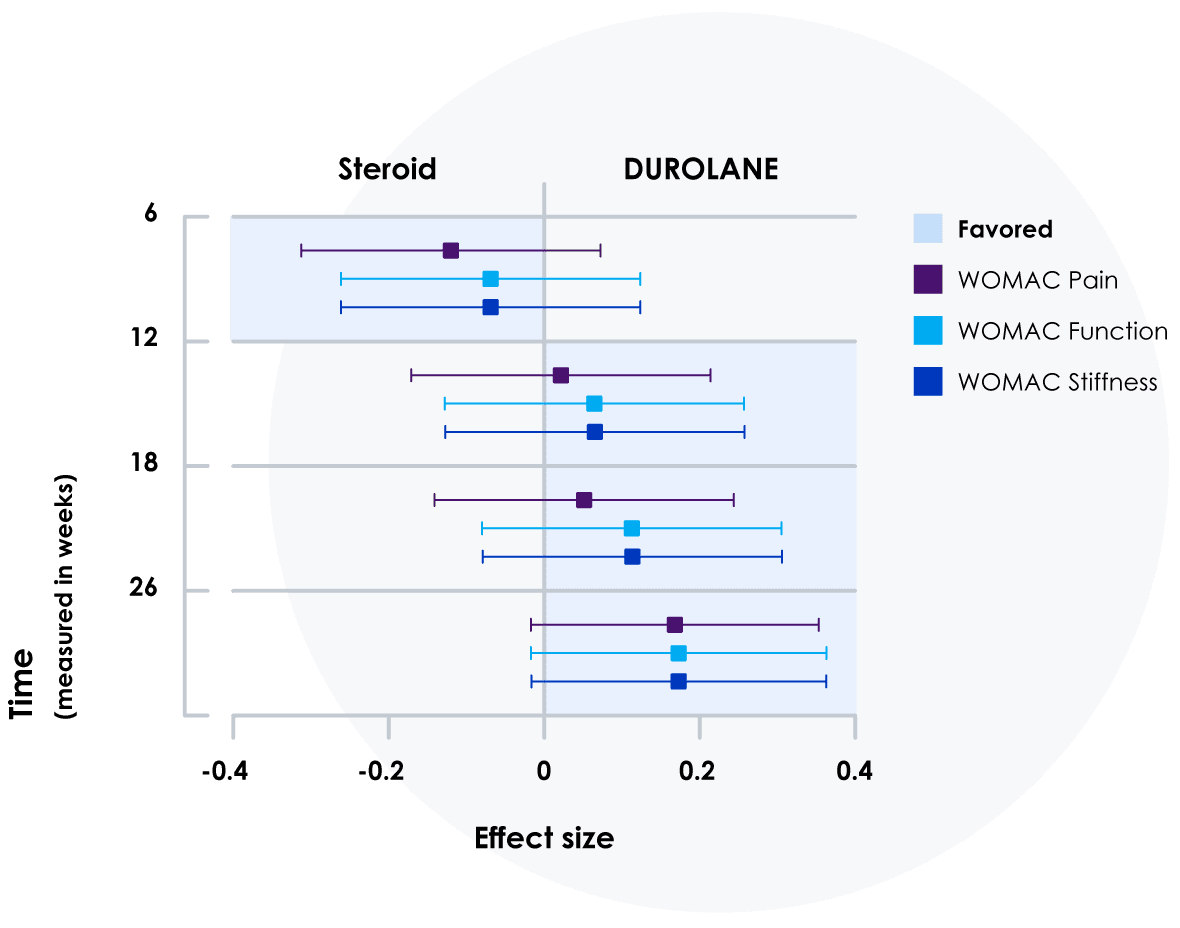
Compared directly to steroids injections,‖24 DUROLANE relieved pain longer.
In a Level 1 study:
- DUROLANE was proven to be noninferior to an intra-articular injected steroid at 6 weeks
- Results favored DUROLANE for reduction in WOMAC pain, function and stiffness scores from weeks 12 to 26
- A significant reduction from baseline to week 26 in WOMAC pain scores was achieved with DUROLANE vs steroid
‖The primary outcome was the WOMAC pain responder rate, defined as at least 40% relative improvement and 5-point absolute improvement from baseline values at 12 weeks.
¶Effect-sizes for WOMAC domains with 95% CIs during the blinded phase of the study.
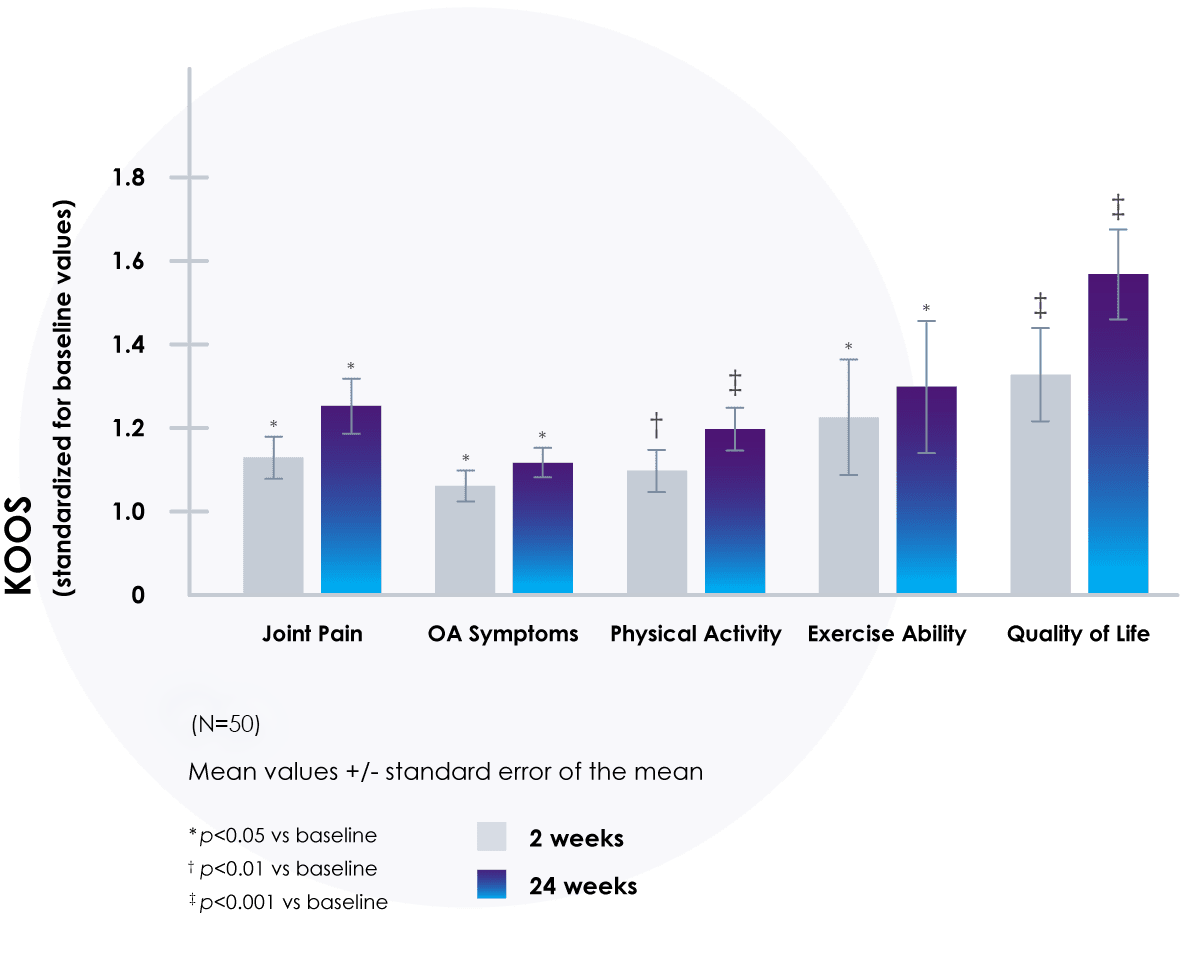
Significantly Improved Quality of Life
A single injection of DUROLANE significantly improved all Knee and Osteoarthritis Outcome Score (KOOS) parameters in as early as 2 weeks, and the improvements persisted for up to 24 weeks.20