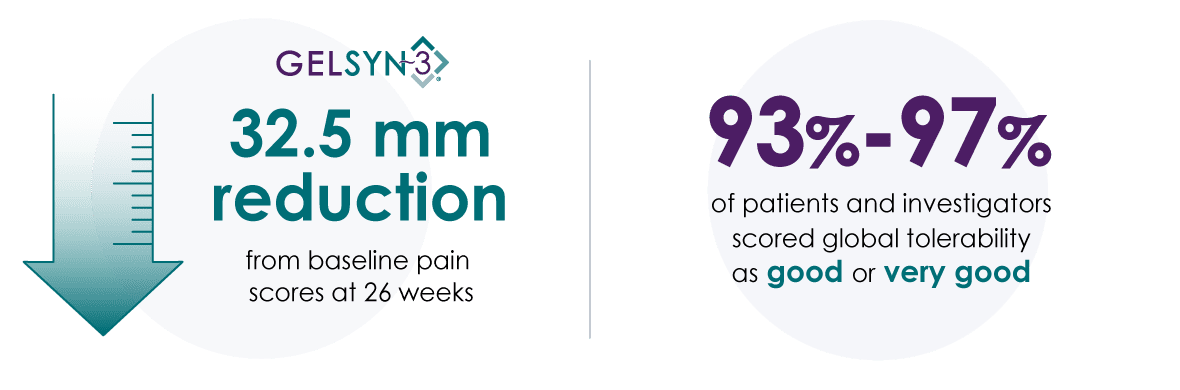
Choose GELSYN-3 for proven outcomes9
GELSYN-3 has efficacy equivalent to a high-molecular-weight hyaluronic acid comparator:
- A 3-injection series of GELSYN-3 (0.8% highly purified, hyaluronic acid of biofermentation origin) or Synvisc® (0.8% hylan G-F 20)
- Patients (N=380) were observed for a period of 26 weeks
- Primary outcome measure: WOMAC pain score at 26 weeks
Synvisc is a registered trademark of Genzyme Biosurgery.
GELSYN-3 also demonstrated improvements in knee function, stiffness, and global pain scores.