This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
In clinical studies, adverse events common to intra-articular treatment were comparable between SUPARTZ FX and saline-treated patients16,22,38,39
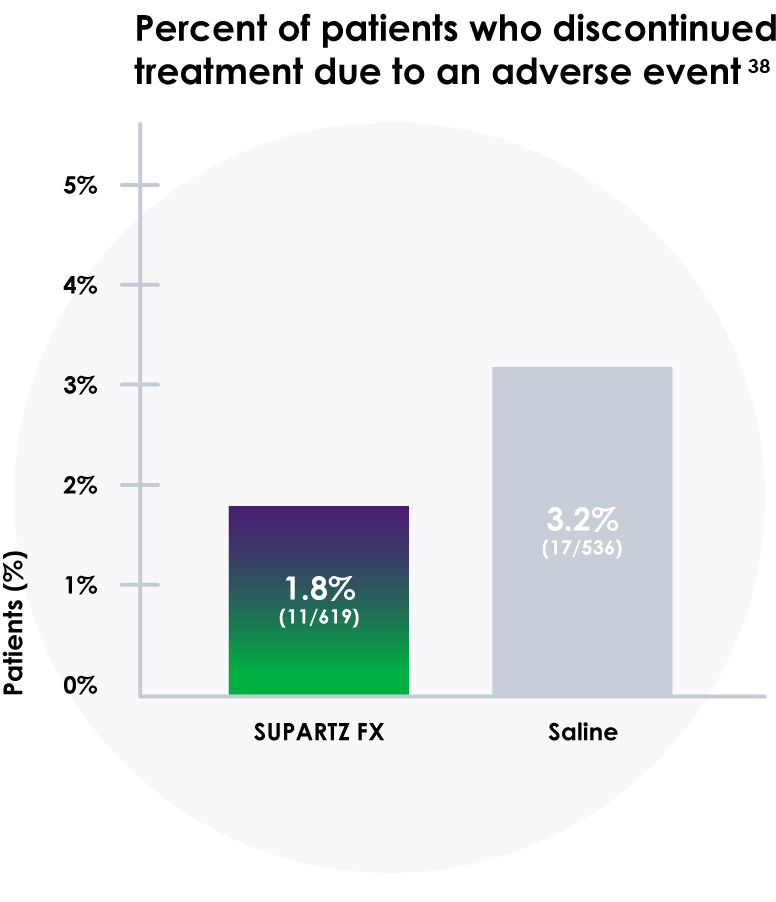
- In these studies, more people discontinued treatment with saline placebo than those who received SUPARTZ FX therapy38
- Pseudoseptic (acute inflammatory) reactions have NOT been reported in 20 prospective randomized controlled trials of
SUPARTZ FX therapy41 - The FDA reviewed data on more than 8,000 patients prior to approving SUPARTZ FX in the United States37
SUPARTZ FX offers a safety profile similar to saline placebo
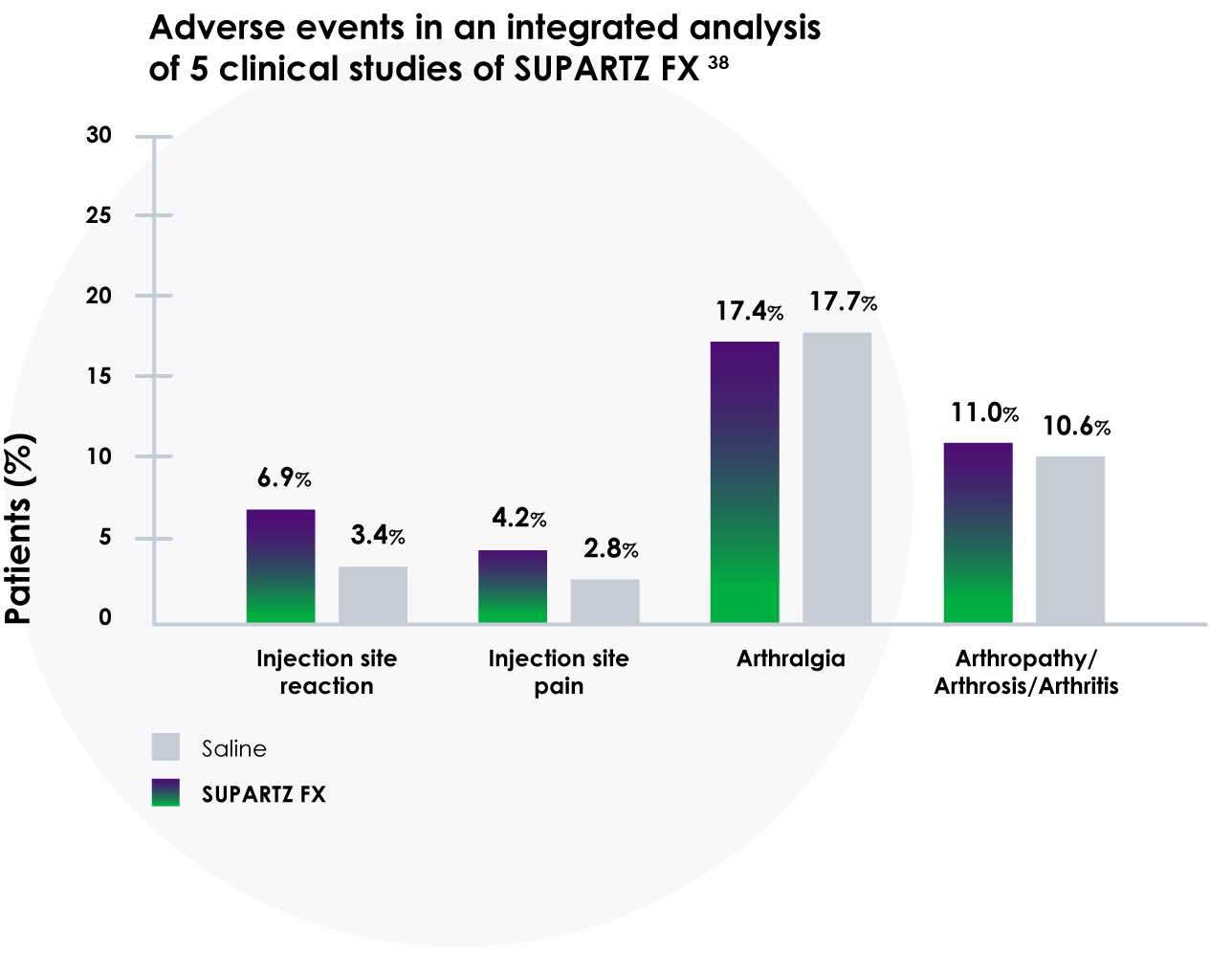
Adverse events occurred at similar rates in patients treated with SUPARTZ FX and those treated with a saline placebo in an integrated analyses of multiple clinical studies. No serious injection site adverse events were reported and no allergic reactions due to SUPARTZ FX were reported.38
- Results of integrated analysis of 5 clinical trials with 1,155 patients showed SUPARTZ FX had adverse events and discontinuation rates similar to saline placebo
- Comparable incidence of adverse events following SUPARTZ FX versus saline placebo16,38
- Discontinuation rates due to adverse events were 1.8% for SUPARTZ and 3.2% for placebo38
- No reports of pseudoseptic reactions in the scientific literature‡41
‡Does not include current prescribing experience.
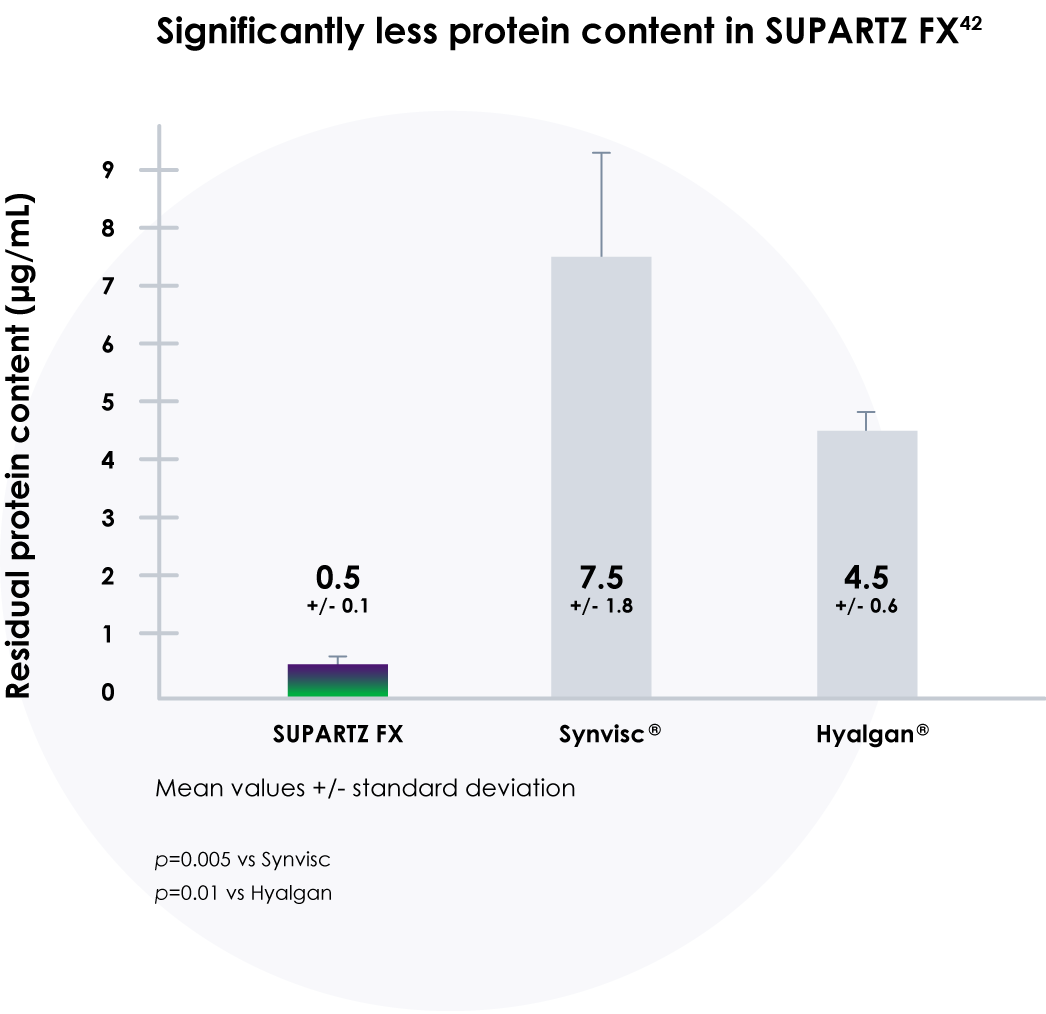
SUPARTZ FX contains virtually no detectable impurities
- A study of impurities in hyaluronic acid (HA) products found that SUPARTZ FX has less protein content than other HA products42
- SUPARTZ FX also had significantly less bacteria endotoxin content and significantly less nucleic acids/DNA content compared with other HA products42
See additional SUPARTZ FX Resources
All registered trademarks are the properties of their respective companies.